Our studies
Our strong confidence in the GASMAS technology and its application in the Neola® device for continuous lung monitoring has been built during the recent years and we have taken several steps to improve both the performance and the clinical acceptance. Read more about our work below.
If you want to know more about the technology, read our white paper.
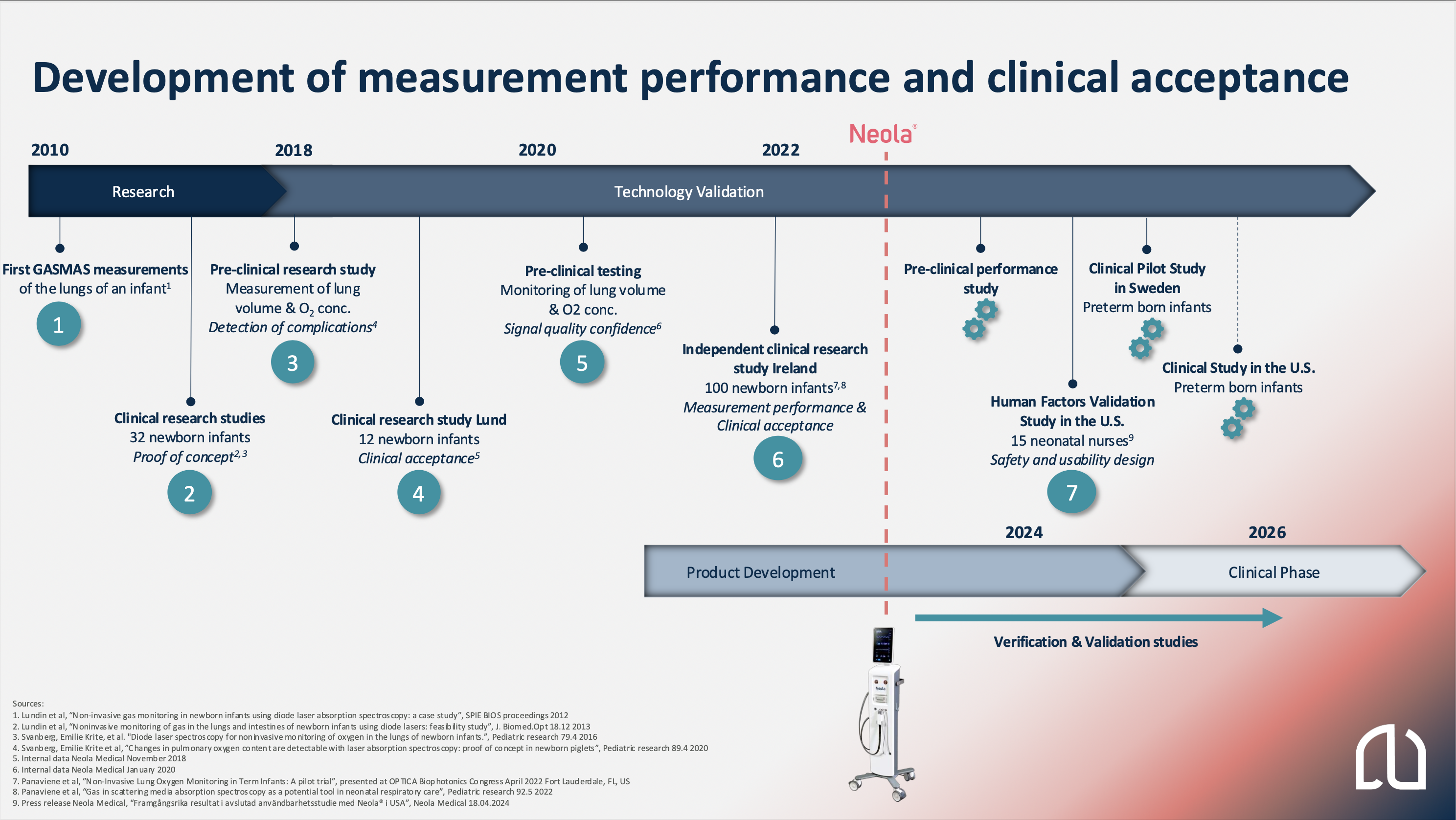
- In 2011, the first gas absorption measurement was performed on one full-term newborn baby at Lunds University Hospital, followed by two more research studies in 2012-2013. In total 32 full-term newborns were measured, and the results demonstrate the feasibility of measuring gas in the lungs of full-term newborns.
- In 2015-2018, an EU-funded project, including pre-clinical and clinical studies on healthy full-term newborns, laid the foundation for the company’s product, Neola®. An additional clinical study involving 12 full-term newborn babies was conducted in Lund. The study demonstrated clinical acceptance of the measurements and the design of the probes, which are attached to the baby’s chest, one of the most important perspectives to consider for this vulnerable patient group.
- In 2018, a pre-clinical study showed the technology’s ability to measure oxygen gas concentrations in the lungs, follow changes in lung volume and to detect respiratory complications.
- The product development was initiated in late 2020, after improvements made to the electronics design in 2019.
- During 2021 and 2022, a large investigator-initiated and independent clinical study was conducted at the INFANT Centre at Cork University Hospital in Ireland. A total of 100 full-term newborns participated in the study, and the results demonstrated that the technology is safe and well tolerated for measuring the oxygen in the lungs. Oxygen detection in the lungs was successfully achieved in all participating babies. This study has further strengthened our confident in the performance of the measurements and the clinical acceptance and Neola®’s future role in Neonatal Intensive Care Unit. Read more about the study below.
- During 2024 Neola Medical conducted Human Factors Validation Study with 15 neonatal nurser in the U.S. and acquired CB certification, meeting partial regulatory requirements for market approval in the U.S. As a result, Neola Medical is transitioning from the technical verification phase to the clinical phase, preparing for clinical studies.
-
Published articles
Previously published articles related to the technology of Neola Medical.
Noninvasive monitoring of gas in the lungs and intestines of newborn infants using diode lasers: feasibility study
Patrik Lundin, Emilie Krite Svanberg, Lorenzo Cocola, Märta Lewander Xu, Gabriel Somesfalean, Stefan Andersson-Engels, John Jahr Vineta Fellman, Katarina Svanberg and Sune Svanberg
Journal of Biomedical Optics, 2013
Diode laser spectroscopy for noninvasive monitoring of oxygen in the lungs of newborn infants
Emilie Krite Svanberg, Patrik Lundin, Marcus Larsson, Jonas Åkeson, Katarina Svanberg, Sune Svanberg, Stefan Andersson-Engels and Vineta Fellman
Pediatric Research, 2016
Comparison of dermal vs internal light administration in human lungs using the TDLAS-GASMAS technique—Phantom studies
Jim Larsson, Dennis Leander, Märta Lewander Xu, Joakim Bood and Emilie Krite Svanberg
Journal of Biophotonics, 2019
Changes in pulmonary oxygen content are detectable with laser absorption spectroscopy: proof of concept in newborn piglets
Emilie Krite Svanberg, Jim Larsson, Martin Rasmussen, Marcus Larsson, Dennis Leander, Sara Bergsten, Joakim Bood, Gorm Greisen and Vineta Fellman
Pediatric Research, 2020
Gas in scattering media absorption spectroscopy as a potential tool in neonatal respiratory care
Jurate Panaviene, Andrea Pacheco, Christoph E. Schwarz, Konstantin Grygoryev, Stefan Andersson-Engels and Eugene M. Dempsey
Pediatric Research, 2022
Evolution of gas in scattering media absorption spectroscopy as a neonatal pulmonary monitoring device
Hemananda Kumar Muniraman, Judith Klein-Seetharaman and Vineet Bhandari
Pediatric Research, 2022
Numerical investigation of the influence of the source and detector position for optical measurement of lung volume and oxygen content in preterm infants
Andrea Pacheco, Baptiste Jayet, Emilie Krite Svanberg, Hamid Dehghani, Eugene Dempsey and Stefan Andersson-Engels
Journal of Biophotonics, 2022
Non-Invasive Lung Oxygen Monitoring in Term Infants: A Pilot Trial
Jurate Panaviene, Konstantin Grygoryev, Andrea Pacheco, Eugene Dempsey and Stefan Andersson-Engels
Biophotonics congress OPTICA, 2022
Laser absorption spectroscopy measurements of different pulmonary oxygen gas concentrations in transmittance and remittance geometry: phantom study
Andrea Pacheco, Jean Matias, Konstantin Grygoryev, Martin Hansson, Sara Bergsten and Stefan Andersson-Engels
Journal of Biomedical Optics, 2023
White Paper: Neonatal Lung Analyzer
Tetiana Kovtiukh and Martin Hansson
Neola Medical AB, 2023
Neola® obtains CB certification and meets partial regulatory requirements for market approval in the U.S.
In June, 2024, Neola Medical announced that Neola®, the company’s medical device for continuous monitoring of the lungs of preterm born infants, has successfully passed the comprehensive and stringent testing against IEC 60601-1 and IEC 60601-1-2 standards. This has resulted in the issuance of an IECEE CB Scheme certificate, which is recognized proof of the safety and essential performance of Neola®, issued by the accredited testing body FORCE Technology. The certificate is internationally recognized in about 50 countries and will form the basis for meeting the FDA’s regulatory requirements.
The CB certificate signifies that Neola® has undergone a rigorous and comprehensive testing and evaluation process at an accredited and independent IECEE testing house, FORCE Technology. The certification for IEC 60601-1 and IEC 60601-1-2 indicates that the product meets specific quality requirements and international standards for medical devices in healthcare, with particularly high demands on safety, essential performance, and electromagnetic compatibility. In practical terms, this means that Neola® has been tested and verified to be safe for use according to the requirements for its application in neonatal intensive care units. This is a crucial piece that the company has secured in preparation for the market approval of Neola® in the U.S.
Human Factors Validation Study with Neola® in the U.S.
In March, 2024, Neola Medical AB (publ) completed the Human Factors Validation Study with Neola®. The study was conducted in Boston, USA, to validate the usability of the medical device Neola®, and the successful results of the study will be included in Neola Medical’s application for market registration approval ahead of the market launch in the U.S.
Evaluation of usability is a regulatory requirement from the the US Food and Drug Administration (FDA) and the goal of the study is to assess how well participants interact with Neola® and demonstrate that it is safe for its intended use by healthcare professionals in neonatal intensive care units. The collected results have been evaluated and the report clearly indicates a usable and safe design. The report will be part of the forthcoming FDA application for market approval of Neola® in the U.S.
The study was led by Custom Medical, global experts in usability studies, and conducted at a testing center in Boston during March 2024. The study’s design has been aligned during a pre-submission meeting with the FDA, and the study has been executed entirely according to plan. The participants in the study consisted of 15 neonatal nurses, with varying levels of experience in the field, from several neonatal intensive care units in the U.S. Neola Medical’s staff were present in Boston to train the study participants in the use of Neola®.
Non-Invasive Lung Oxygen Monitoring in Term Infants (NIOMI)
Tyndall National Institute at University College Cork in Ireland (UCC) aquired a research system with the Neola®-technology from Neola Medical AB (publ) in 2021 and started a large independent clinical research study on newborn infants at the research facility at Cork University Maternity Hospital. The study was conducted in cooperation with The Irish Centre for Maternal and Child Health Research (INFANT) at UCC and included in total 100 newborn babies.
The aim of the study is to investigate the possibility to give real-time information about the lung function in newborn babies. The study examines the influence of the location of the Neola®-probes on different areas of the infants’ chests, which can provide valuable clinical information for using Neola® at neonatal intensive care units by doctors and nurses. The principal investigator of the study is Professor Eugene Dempsey, Horgan Chair in Neonatology, INFANT Centre, University College Cork (UCC), and he is supported by the BioPhotonics team at Tyndall.
Results and conclusions from the first 50 newborn infants were presented on the 25th of April 2022 by clinical researcher and neonatologist Jurate Panaviene at the OPTICA Biophotonic Congress: Biomedical Optics in Florida, the U.S. Read the press release about it here.
During the fall 2022, the clinical study was finalized after successful recruitment of 100 babies. The results and conclusions from the 100 newborn infants were presented at the jENS conference in Italy on the 23rd of September 2023, by clinical researcher and neonatologist Jurate Panaviene.
Pre-clinical and clinical studies on healthy full-term newborns
In 2015-2018, an EU-funded project, including pre-clinical and clinical studies on healthy full-term newborns, laid the foundation for the company’s product, Neola®. An additional clinical study involving 12 full-term newborn babies was conducted in Lund. The study demonstrated clinical acceptance of the measurements and the design of the probes, which are attached to the baby’s chest, one of the most important perspectives to consider for this vulnerable patient group.
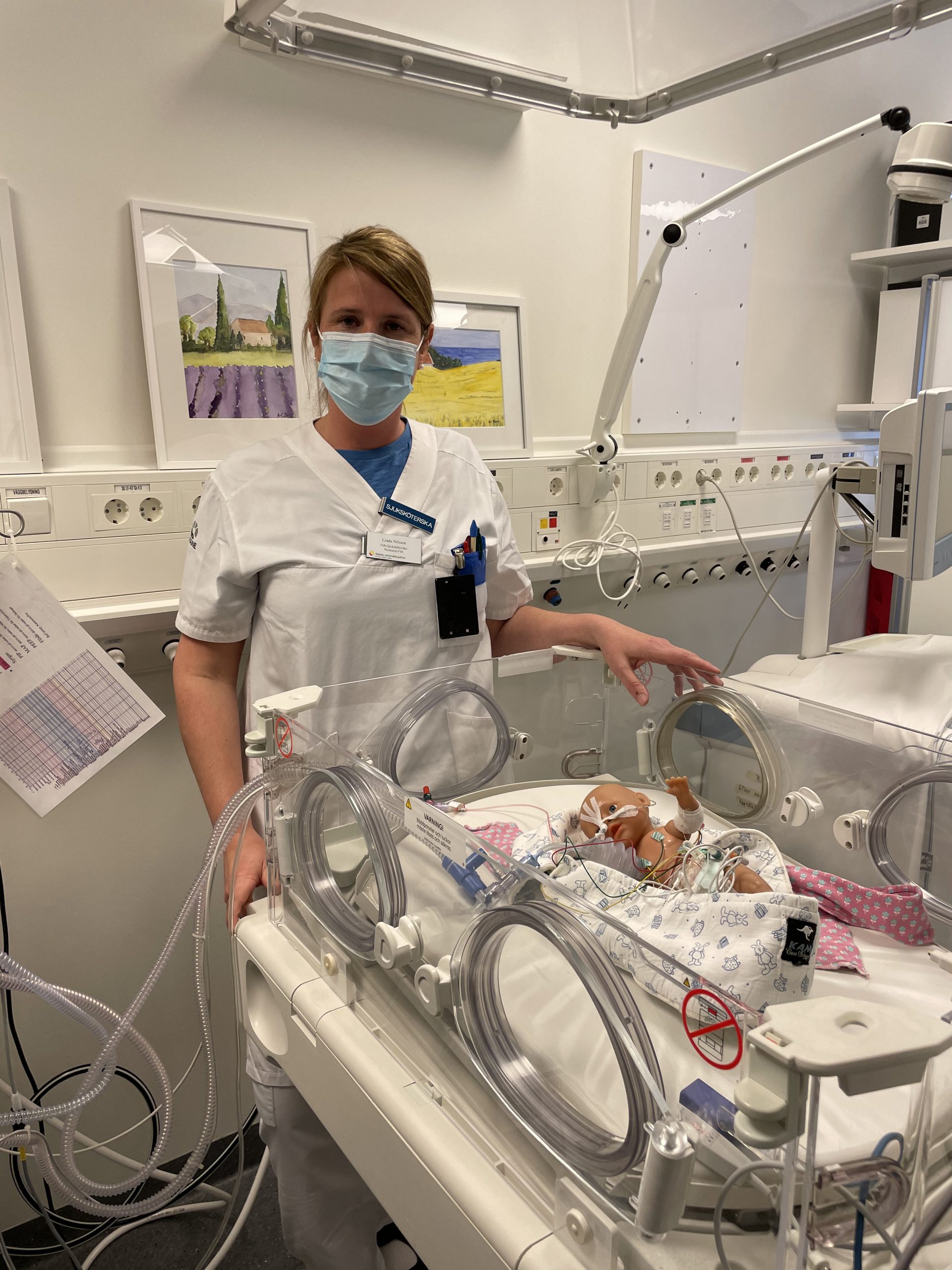
MD Ph.D. Emilie Krite Svanberg and MSc. Dennis Leander, during clinical pilot study on healthy full-term newborn babies at Skåne University Hospital in the fall of 2018.
Research work at Neola Medical
As part of the development process at Neola Medical, internal usability and performance studies is performed with nurses, neonatologists, dolls and with animal models to ensure performance, safety and usability.
Information about risks and uncertainties at our website here.
For Investors
We are on a journey to revolutionize neonatal intensive care
Learn more about investing in Neola Medical