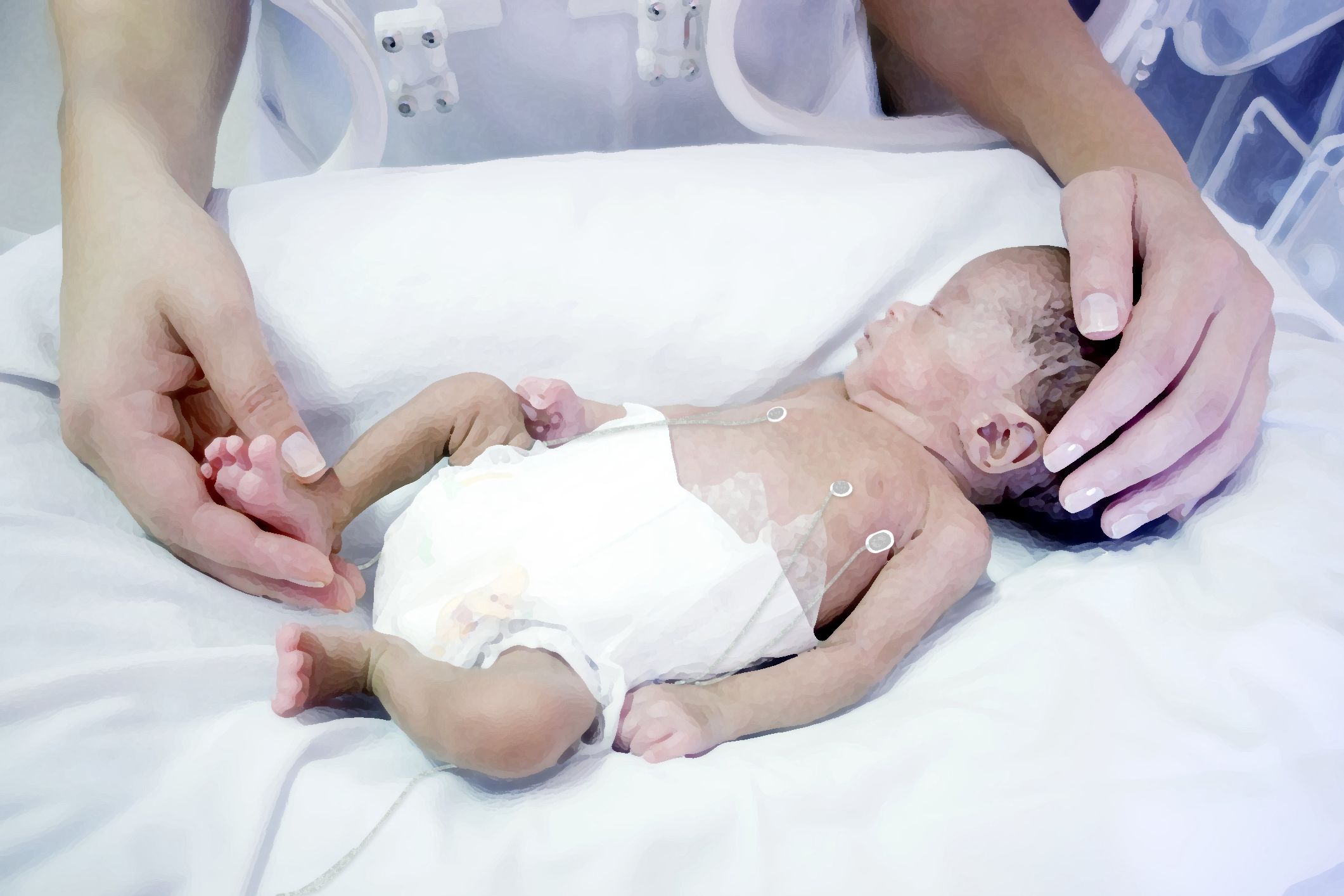
Caring for our most vulnerable patients
Revolutionizing neonatal care
Every year, approximately 15 million babies worldwide – about one in ten – are born preterm (prior to pregnancy week 37). Although significant progress has been made to increase the survival rate of preterm born babies over the past 50 years, approximately 1.1 million babies still die every year.
The most common cause of death for preterm born babies is respiratory distress syndrome (RDS), a serious lung condition whose complications can affect up to 80 percent of babies born extremely preterm. The methods used by neonatal health care professionals today involve manual observation with help from e.g. chest x-rays and blood samples. These methods do not provide continuous information for decision making. They can also cause serious discomfort and pain for patients, a higher risk for developing cancer later on in life and high health care costs.
Neola® – continuous, safe and noninvasive lung monitoring for preterm born babies
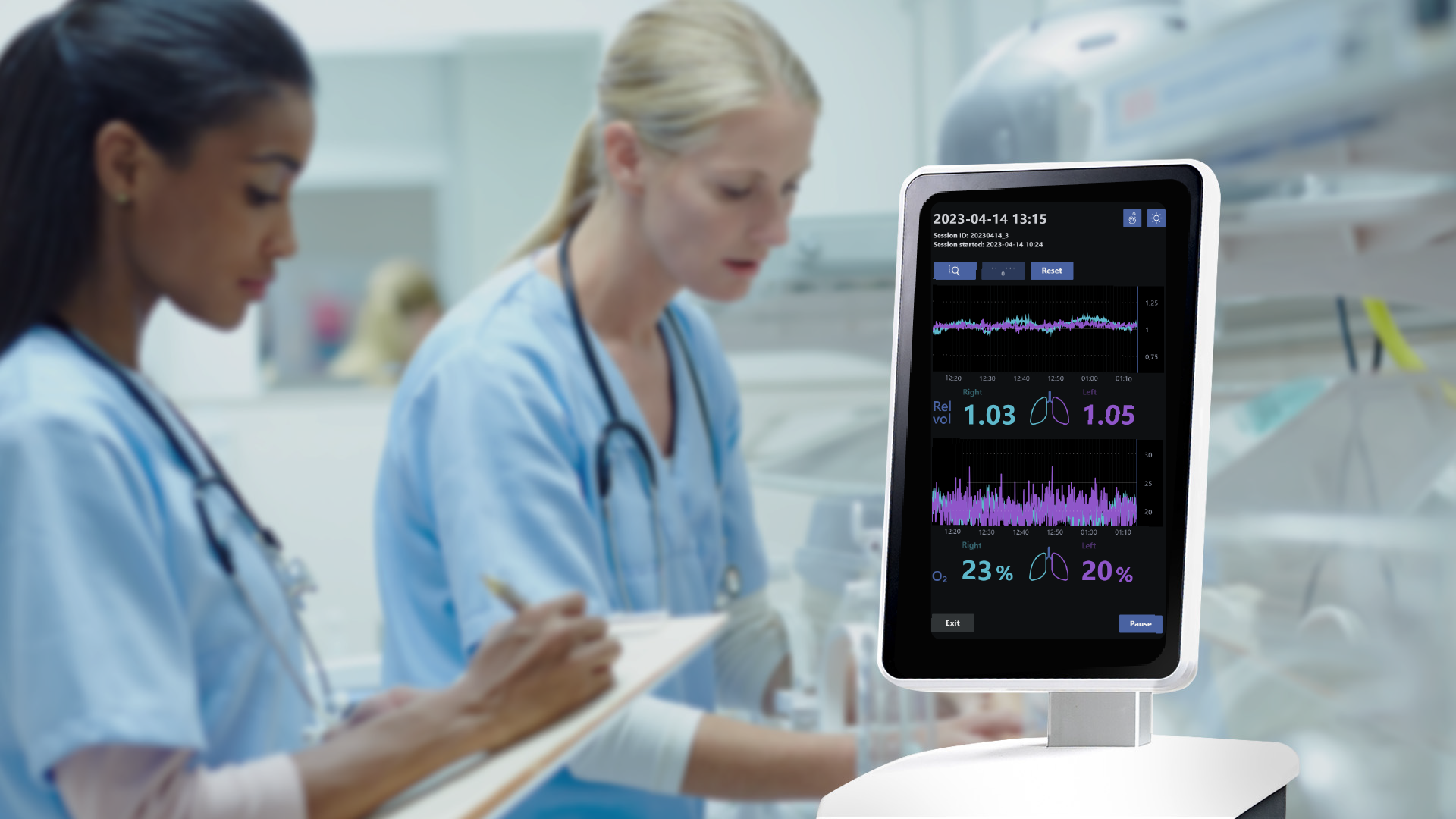
Neola Medical’s Neola® can offer a safe and non-invasive way to continuously measure lung volume changes and oxygen gas saturation in the lungs of preterm born babies with the possibility of instantly detecting complications such as an obstructed airway or a misplaced tracheal tube. This means that health care personnel are alerted to problems in real time and can treat patients right away.
Neola® uses a biomedical application of GASMAS* technology originally developed by researchers at the Division of Atomic Physics at Lund University. Small and lightweight emitter and detector probes are positioned on the skin. Near-infrared light is sent through the chest cavity, where a small portion is absorbed by water vapor and oxygen gas molecules in the lungs. The detector probe detects some of the light that travelled through the tissue and the signal shows how much of the light that has been absorbed by the gas molecules. Using this data, Neola® can register small changes related to lung volume and oxygen gas concentration. The probes can be easily removed and repositioned, avoiding discomfort and stress for the baby.
*Gas in Scattering Media Absorption Spectroscopy (GASMAS)
A gamechanger in the pediatric landscape
Development of medical technology has almost exclusively focused on devices for adults. The FDA has acknowledged this discrepancy and highlighted the need for more pediatric medical devices. The FDA has therefore taken concrete steps to incentivize development of medical devices for children and babies.
Several factors suggest that Neola Medical (publ) with Neola® has the potential to evolve and upgrade the care of preterm born babies: increased regulatory focus on developing pediatric medical devices, the United Nations Global Agenda 2030 partial goal of reduced neonatal mortality caused by preventable conditions (Goal 3.2), the positive investment climate of the medical technology sector as well as a historic Swedish tradition of leading the way in innovations related to medical technology and neonatal care.
Information about risks and uncertainties at our website here.
CEO Hanna Sjöström and Dr. Janene H. Fuerch, Co-Director Impact1. Click to watch the welcome speech to the Stanford Impact1 program by Chair of Executive Board
and Founding Director, James Wall.
“I truly appreciate this as a neonatologist, as I frequently encounter cases of pneumothorax where a lung can collapse, and we do not detect it until vital signs begin to change. X-rays are not always particularly helpful. Gaining a real understanding of oxygenation and how well the lungs are expanded could therefore be extremely valuable for our patients.”
– Dr. Janene H. Fuerch, Co-Director Impact1
Stanford Impact1
During 2023, Neola® was recognized by leading experts in neonatology at Stanford University as a promising innovation with the potential to upgrade neonatal intensive care from the first day in the clinic. As a result, Neola Medical was selected as a Stanford Impact1 company, receiving support through the Stanford Impact1 program.
In the program, Neola Medical gets access to company development from Stanford and Silicon Valley experts in development for medical technology products, regulatory experts and close collaboration with leading doctors in neonatal intensive care as well as the FDA’s own pediatricians and regulatory experts.
Dr. Janene H. Fuerch, MD, Co-Director of Impact1, Clinical Associate Professor of Pediatrics, Division of Neonatal and Developmental Medicine at Stanford University School of Medicine, Medical Director, Neonatal ECMO at Stanford Children’s Hospital, Palo Alto, USA
Stanford Impact1 Program
Stanford is one of the world’s leading universities in research on preterm born babies and connected to the hospital that is ranked number one in neonatal care in the U.S. Stanford launched the Impact1 program with the vision of improving the health, safety and quality of life of pediatric and maternal patients globally. The name Impact1 comes from the purpose to promote innovations that provide precisely ”impact from day 1”. The FDA is supporting the program by awarding five years of funding to the Stanford Pediatric Device Consortium (PDC) in 2023, with the aim of supporting advanced technological innovation to reach the vulnerable pediatric patient group as quickly as possible.
CEO Hanna Sjöström at exclusive Pediatric Medtech CEO Summit 2024 by Stanford Impact1
“Being part of this exclusive network of MedTech entrepreneurs, investors, and leading pediatric and neonatology specialists is invaluable. It is truly inspiring to see our vision for transforming neonatal intensive care resonate with such influential key opinion leaders, who recognises the critical role Neola® could play in improving outcomes for preterm born babies. The support by Stanford will be of great importance for our upcoming launch of Neola® in the U.S.”
– CEO Hanna Sjöström
For Investors
We are on a journey to revolutionize neonatal intensive care
Learn more about investing in Neola Medical